
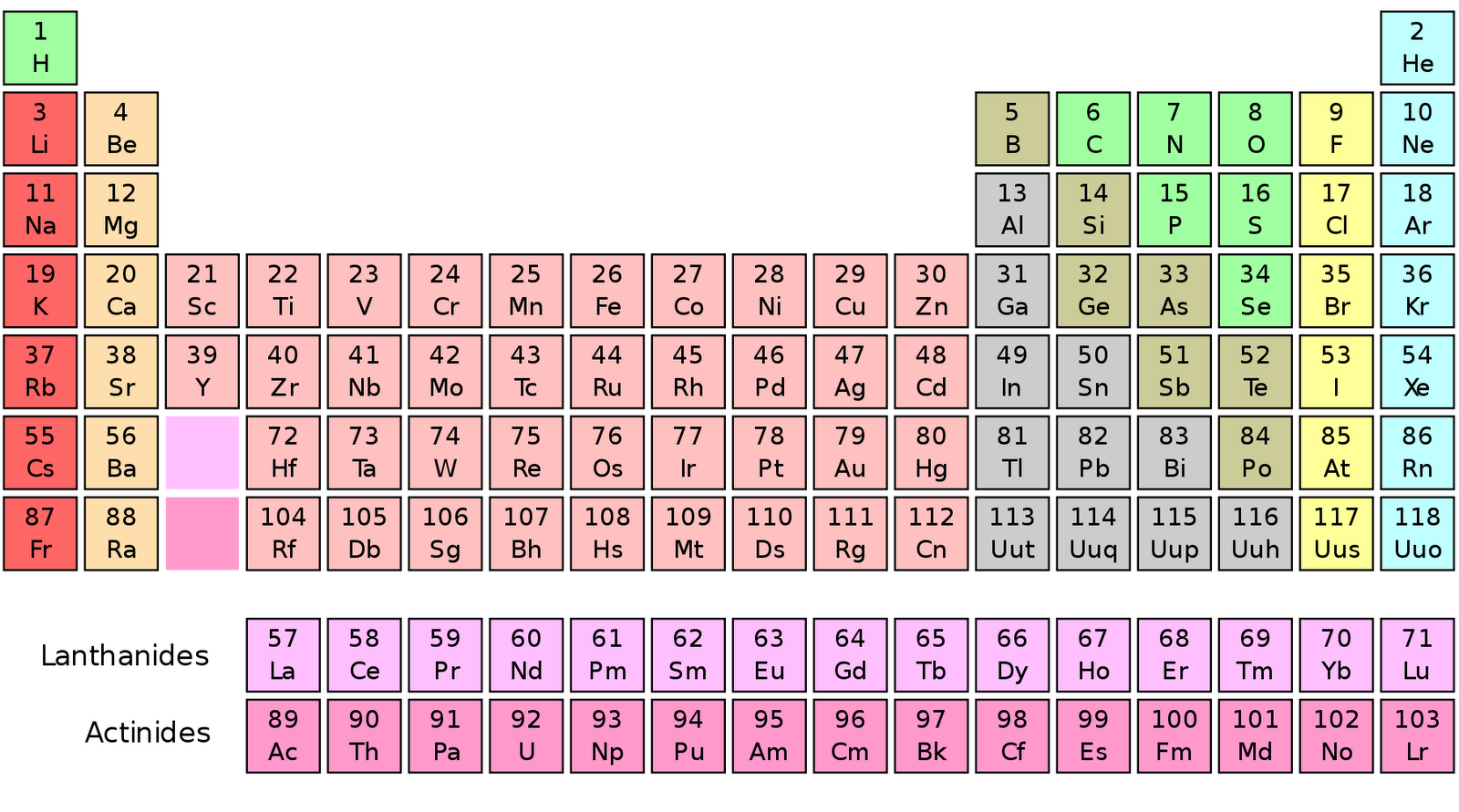
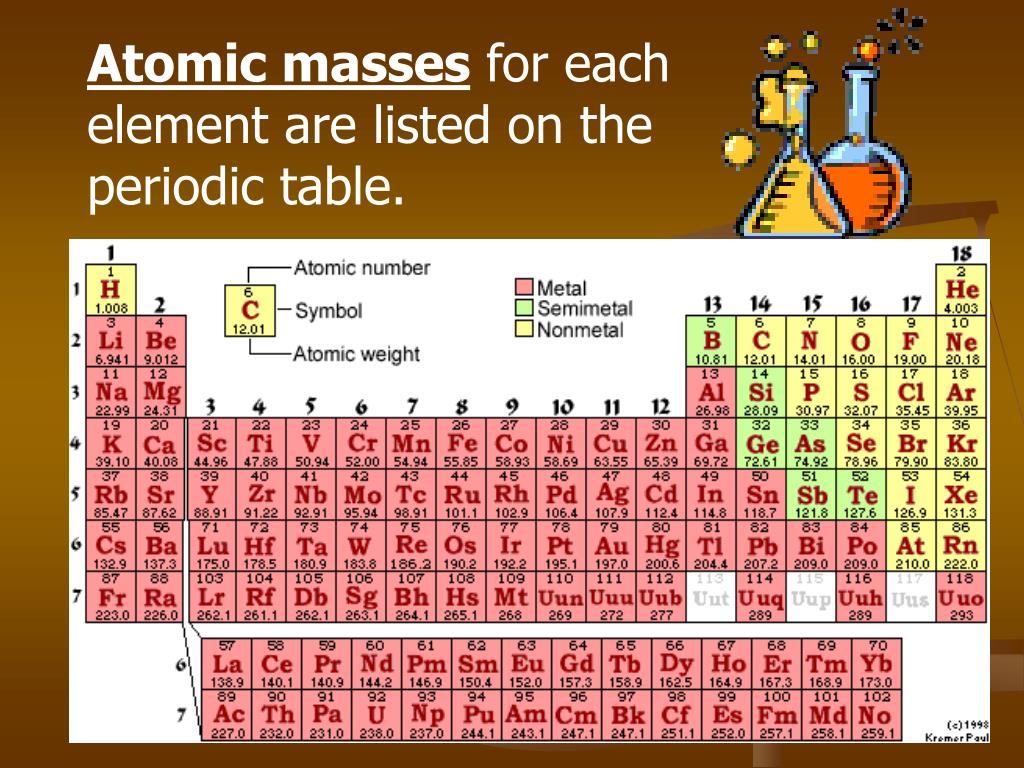
Moreover, the number of protons never changes for an element. Since oxygen has an atomic number of eight, there must be eight protons total. The atomic number is located above the element’s symbol. According to the periodic table, oxygen has the atomic number eight. The number of protons in an atom is equal to the atomic number of the element. The easiest way to find the atomic number, is to look on a periodic table, the atomic number is in the upper left corner, or is the largest number on the square. The atomic number of an element is simply the number of protons in its nucleus. Element: A pure substance that cannot be broken down into a simpler substance by chemical means.Atomic Number: Number of protons present in an atom.Atomic Mass: A weighted average of the number of neutrons and protons present for all isotopes.Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom.Protons: Positively charged subatomic particles located in the nucleus of an atom.If you enjoy this tutorial, be sure to check out our others! Covered in other articles In addition, you will learn about the different subatomic particles.
Periodic table with atomic number and atomic mass how to#
In this tutorial, you will learn how to find and calculate the number of protons, neutrons, and electrons in an atom or element.


 0 kommentar(er)
0 kommentar(er)
